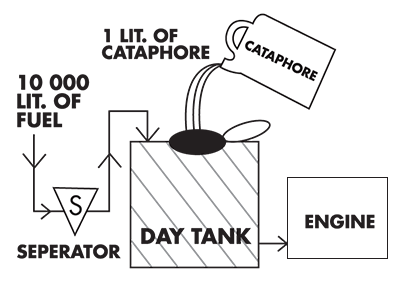
Cataphore
Cataphore is a fuel oil treatment compound totally organic.
| NON TOXIC / NON DANGEROUS |
|---|
DISPERSANT ACTION
DETERGENT ACTION
STABILIZING ACTION
DEMULSIFYING ACTION
In light grade diesel fuel water haze formation resulting from entrained moisture is minimized.
ANTI-CORROSIVE ACTION
IMPROVED COMBUSTION
Cataphore
Cleans fuel oil distribution system
Cataphore
Disperses water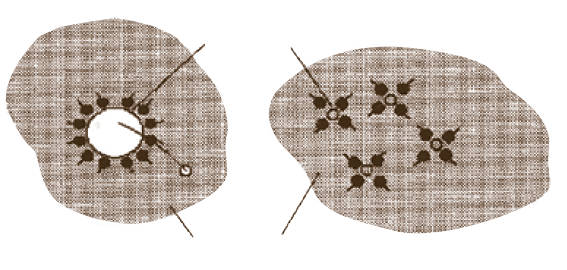
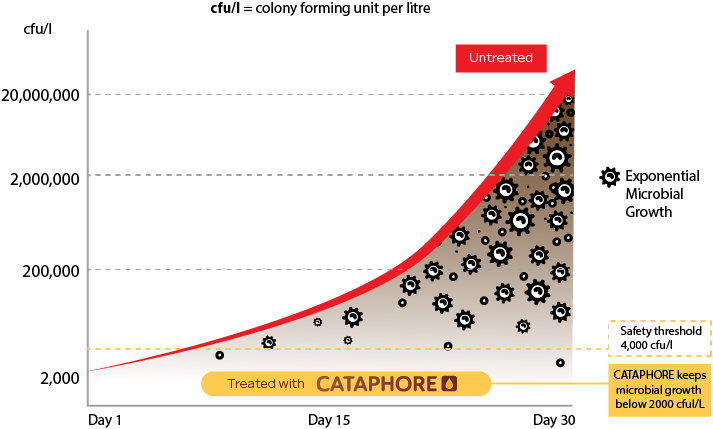
Microbial growth (cfu/l) in biofuel over time
Advantages
As users are experiencing, TODAY’S RESIDUAL FUEL OIL CONTAINS a greater deal of SOLIDS IN SUSPENSION. Many solids, such as ASPHALTENES, TEND TO AGGLOMERATE forming large particles THAT PRECIPITATE AND DEPOSIT at the bottom of reservoir to form sludge or hindering proper atomization with results of poor combustion, carbon, smut and soot.
CATAPHORE FORMS A FILM OR COATING UPON THE PARTICLES which prevents them from joining or contacting other particles. THUS, THE PROTECTIVE BARRIER, created by CATAPHORE’s homogeneous action, PREVENTS COAGULATION OR AGGLOMERATION and produces a stabilization of the particle size. The smaller particles are more easily dispersed and less subject to settling. As they pass completely through strainers, they are easily burned, improving combustion results.
All bunker fuels contain insoluble solids in suspension that have a tendency to coalesce and precipitate. Moreover, both light and heavy fuel oil are subject to oxidation, producing solids that have, again, a tendency to deposit at the bottom of reservoir. Sludge, in fact, consists of those insoluble organic compounds, carbon particles, various polymerized and organic compounds and sediments, all of which are present in today’s residual blending. Combustion is adversely affected because the burner is trying to handle two types of material at the same time : the liquid oil and the solid insoluble substances. BY REDUCING THE INTERFACIAL TENSION of the oil across the burner tips, and dissolving solids, resulting in as much as a seven-fold improvement in atomization, CATAPHORE triggers better firing conditions. Moreover, CATAPHORE also rapidly eliminates sludge formation.
Symptoms of sludge accumulation in the distribution system include loss of fuel flow or fuel pressure, unsteady flame and the need for excessive preheating of fuel oil for proper atomization. CATAPHORE COATS ALL SURFACES WITH A MONOLAYER OF SURFACTANT, such as the inside walls of reservoir, the interior of tubing, the strainers and nozzles. ANTI-SCREEN CLOGGING TEST, ran according to Socony Mobil Analytical Method 530-64, HAS PROVED THAT CATAPHORE DISPERSES MORE THAN 90% OF SLUDGE DEPOSITS, illustrating therefore CATAPHORE’S EXCELLENT DISPERSANT QUALITIES.
Cleaner storage tank, strainers and injectors, cleaner engine means better control over labour and maintenance costs. Total savings, resulting from reduction of fuel consumption and reduction of labour and maintenance costs, may range from 8 to 10% on annual bill. By improving the compatibility between residual and light oil in today’s blending and dissolving solids, CATAPHORE PREVENTS THE ENTIRE EQUIPMENT FROM COAGULATED SLUDGE.
By producing an unrestricted liquid flow, CATAPHORE enables the fuel oil to be better atomized and burned. Thus, the system is clean, the correct atomization is obtained and good combustion results. The net result is a smoother operation.
CATAPHORE REDUCES VANADIUM AND SODIUM DEPOSITS. IT ALSO LIMITS EXTENSIVELY CORROSION EFFECTS AND FOULING IN POST-COMBUSTION AREAS.
CATAPHORE DISSOLVES SOLIDS in residual and diesel fuel oil making it more homogeneous, Thus REDUCING DEPOSITS. Updated statistics show that CATAPHORE REDUCES MORE THAN 90% of VANADIUM ACCUMULATIONS, extending therefore equipment performance and overall life of the system.
Better combustion means elimination of much of the soot and smoke. Reduction of vanadium deposits slows down formation of SO3 which when in contact with water produces corrosive H2SO4.
The additional following benefits can be obtained by the use of CATAPHORE :
- Lower pre-heating temperature needed
- Injectors, pistons and rings free of carbon deposits
- Easier fuel oil pumpability
- Reduction of soot and carbon emissions
What is Cataphore?
CATAPHORE is the strongest oil soluble detergent available.
CATAPHORE IS A FUEL OIL TREATMENT COMPOUND TOTALLY ORGANIC.
CATAPHORE is a semi-polar active organic compound especially synthetized to minimize sludging and overcome incompatibility in residual and diesel fuel operations.
Basically, CATAPHORE is a t’ synthetized molecule of aliphatic and cyclic amines that acts as AN HOMOGENIZER AND A DEMULSIFIER. CATAPHORE removes residues which adhere to solid surfaces.
CATAPHORE molecules are available in abundance. 1 LITRE OF CATAPHORE CONTAINS 1024 molecules. All those active molecules help keep asphaltenes in suspension in the fuel oil. It comes in an EASY TO USE LIQUID product designed specifically for bunker and diesel fuel oil operations. IT CONTAINS NO METALS and POSSESSES EXCELLENT SELF-DISPERSANT PROPERTIES, eliminating the need for complicated dosage equipment.
CATAPHORE IS CHEMICALLY STABLE AND PRESENTS NO STORAGE PROBLEMS. It does not freeze in cold weather, nor does it decompose during storage.
As a fuel treatment compound (ASHLESS STABILIZER PACKAGE), CATAPHORE acts in residual and diesel oil as an homogenizer in dissolving solids in suspension in fuel oil as well as by reducing the interfacial tension in fuel oil.
CATAPHORE causes a physical rather than a chemical reaction when added to oil and therefore does not change the chemistry of the treated fuel. In fact, CATAPHORE’s fuel treatment represents “THE MOST ADVANCED STATE THAT MODERN TECHNOLOGY HAS DEVISED”.(1)
(1) “Optimising the performance of fuel treatment materials”, Ing. Z. Polak, reprinted from “The Motor Ship”, Vol 53, page 165, July 1972.
Why Cataphore?
Historically, there has always been a lower price associated with the bunker market in relation to other petroleum products. This explains why refiners have tried to find new ways of minimizing the yield of residual oil and to use it as a catch-all for the heavy ends of various processing operations. In other words, the increased demand for lighter, more profitable fuels has focused interest on squeezing the most out of the residual oil or “bottom of the barrel”. Being sold at poor returns, the bunker fuel oil is still manufactured, but the actual trend is toward reducing its consumption.
The residual oil produced by the straight run method, nowadays, becomes the input for further processing. In other words, the “RESIDUE” left over, after all the “goodies” are distilled, IS THEN BLENDED BACK, in most cases, WITH THE LEAST VALUABLE “cutter stock” available in the refinery to meet viscosity specifications.
Some of the additional processes include : thermal cracking, catalytic cracking, hydrosulphurizing, hydrocracking and cooling. All these processes have one thing in common : they all degrade the bunker fuel oil quality. THE END RESULT IS A FUEL THAT HAS A TENDENCY TO SMOKE AND WHICH REQUIRES MORE ATTENTION AND MAINTENANCE. In heavy fuel oil, these processes remove the solvent from the fuel oil, thus producing insoluble solids in suspension. In light fuel oil, the cracked products, added to a blend of straight run, contain more aromatic hydrocarbons which have a tendency to smoke with more olefinic hydrocarbons subject to oxidation or polymerizatlon with end results of gums and bacteria.
When vanadium and sodium are wrap up in the agglomeration of asphaltenes they are corrosive and cause many problem during combustion. This new distribution of a barrel of crude oil, between distillate and residual fuels creates problems unknown in the past, but which now must be faced by today’s light and heavy fuel oil consumers.
As the quality of fuel diminished, fuel related breakdowns increased to a point where many users became alarmed. Thus, fuel oil ADDITIVES became the object of serious on-going research in order to reduce maintenance costs and energy consumption. Although research was carried on by the industry itself, many observers were skeptical as to the value of these products when they were first introduced. Even today, many still are. Metallic components used in the manufacturing of currently sold additives explain, partly, this lack of enthousiasm.
MOST OF THE ADDITIVES SOLD ON THE AMERICAN MARKET (more than 200 products) were tested by the U.S. Environment Protection Agency. All of them PROVED TO CONTAIN METALS OR METALLIC COMPOUNDS WHICH SIGNIFICANTLY REDUCE THEIR EFFECTIVENESS. The results of the study show that “the use of metallic additives causes a high concentration of metal compounds in the fuel gas, and the possible toxicity of these new emissions makes the use of these additives very questionable”(1). The results clearly showed that most of the metallic additives could not trigger real savings nor could they be efficient in reducing pollutant emissions.
In fact, METALLIC ADDITIVES generally ACT AS IGNITION DELAYERS in diesel fuels. Moreover, there is much evidence that metals, such as copper, zinc, manganese, iron and lead, when used in bunker fuels, may have harmful side effects on cylinder lubricant conditions. Finally, it has been shown that even a low concentration of lead in fuels promote high temperature corrosion of nickelalloyed materials used for exhaust valves in diesel engines. Existing data indicate that the presence of metals or metallic compounds in additives hinder substantially their effectiveness, as it can also affect adversely the lubricating qualities of the oil. Almost all fuel oil additives on the market contain metallic additions for various combustion improvement reasons. Since “CATAPHORE” CONTAINS NO METALLIC ADDITIONS whatsoever, THE PROPLEMS related to metallic compounds ARE TOTALLY ELIMINATED.
(1) Effects of Fuel Additives on Air Pollutant Emissions, G.B. Martin, D.W. Pershing lt al, U.S. Environmental Protection Agency, June 1971.
How Cataphore works
Results with and without CATAPHORE
| Combustible (FUEL OIL WITH CATAPHORE) | Unit | Results without Cataphore | Results with Cataphore | ASTM Analytical Method |
| Cinematic Viscosity at 50° C | SF | 235 | 227 | D-445 |
| Water | % Vol | 0.15 | 0.05 | D-95 |
| Asphaltene | Weight % | 10.0 | 4.2 | |
| Deposit | Weight % | 0.05 | 0.02 | D-473 |
Report on viscosity in shear
Object
This study was performed to investigate the influence of an additive designated CATAPHORE on the viscosity of crude oil (bunker) under moderatly high shear rates and temperature.
Materials
CATAPHORE: Supplied by requestee and used as such.
#6 BUNKER OIL : Supplied by requestee.
Methods
The bunker viscosities were measured using a standard FANN viscometer (rotating inside cylinder, measuring torque on outside cylinder). The apparatus was standardized as described by the manufacturer and tested by measuring the viscosity of ethylene glycol at 25°C; the value found was 20 centi poise, comparing favorably with 19.0 c.p. litterature value at 20°C. Because of the lack of accurate reference liquids at high viscosity (e.g. near 300 c.p. the results given below are precise on a relative basis only. The temperature of the oil was maintained at 67°C ± 0.2°C throughout the experiments by means of a water jacket and a circulating thermostat.
Results
The torgue angle readings (Ø) were converted into corresponding viscosities using the relationship:
n = s x Ø x f x c
where s is a speed factor, f, the torsion spring factor and c is a rotorbob constant dependent on the diameters of the outside and inside cylinders. The viscosity of #6 Bunker oil at 67°C under shear rate of l9 cm/sec ( 100 rpm) was determined as 230 c.p. and taken as reference (unit value). All other viscosities are reported relative to the latter in table 1
| Table 1. | Relative Newtonian Viscosities (n) of # 6 Bunker Oil at 67°C and in the presence of CATAPHORE. |
| RPM | shear rate cm/sec |
Bunker #6 | # 6 Bunker with CATAPHORE | |||
| Con.: CAT. nrel | Con.: CAT. nrel | |||||
| 100 | 19 | 1.00* | 0.02% | 0.95 | 0.2% | 0.90 |
| 200 | 38 | 0.99 | 0.02% | 0.95 | 0.2% | 0.91 |
| 300 | 57 | 0.97 | 0.02% | 0.95 | 0.2% | 0.92 |
* Newtonian viscosity of #6 Bunker measured as 230 cp; all other values given relative to the latter
Estimated uncertainties on nrel : 0.02
Conclusions
The addition of low levels of CATAPHORE produces measurable reduction of bunker oil viscosities under all conditions tested here and the effect if larger than the dilution effect of the solvant in the CATAPHORE additive.
Chimiste
Report on atomization(U.S. Bureau of Mining 5113)
Report on the Atomization of light and heavy fuels in the combustion chamber test on numbers or droplets in systems before and after treatment of fuel with Ivesco’s (Cataphore)
1. Introduction
We received a sample of app. 100 cm in a sealed plastic bottle labeled Ivesco’s (Cataphore). We received two sealed containers 5 liters each:
- Light fuel 200 sec/Redwood 1
- Heavy fuel 1500 sec/Redwood 1
2. Equipment Used
A Sunstrand Fuel burner pump was connected to a container with heat control and pressure gauge and a S 150/3/15/18 injection valve. The atomized stream of oil droplets was led through a timing apparatus 1/60 to 1/1000 sec to a screen. According to the findings issued in the US Bureau of mining 5113 test, the number of droplets reaching the screen for treated and untreated oil and the diameter of these droplets in average are the significant values for an atomization support ability.
The light fuel was preheated to 50°C and the timing gradually decreased from 1/100 of a second always another 1/100 second until a value of 1/1000 of a second was reached. The treatment concentration by Ivesco’s (Cataphore), 1 part of Cataphore to 10000 parts of fuel.
The heavy fuel was preheated to 80°C and the same timings were set. This was repeated 3 times with treated and untreated oil. The results show as follows, using a treatment concentration by Ivesco’s (Cataphore) 1 part of cataphore to 10000 parts of fuel.
LIGHT FUEL
| TIMING SEC. | LIGHT FUEL UNTREATED NO. OF DROPLETS |
LIGHT FUEL TREATED NO. OF DROPLETS |
||||||
| Test 1 | Test 2 | Test 3 | Total | Test 1 | Test 2 | Test 3 | Total | |
| 1/100 | 2800 | 2715 | 2970 | 8485 | 8530 | 9360 | 8160 | 26050 |
| 200 | 2410 | 2470 | 3010 | 7890 | 7180 | 6680 | 7430 | 23270 |
| 300 | 2100 | 2070 | 2350 | 6520 | 6700 | 6680 | 5943 | 20810 |
| 400 | 1630 | 1815 | 1720 | 5165 | 6700 | 7150 | 6840 | 20690 |
| 500 | 1315 | 1420 | 1514 | 4249 | 6400 | 6190 | 6620 | 19210 |
| 600 | 1208 | 1060 | 1330 | 3590 | 6115 | 6230 | 6150 | 18495 |
| 700 | 1070 | 1130 | 1050 | 3250 | 5810 | 5630 | 5960 | 17400 |
| 800 | 930 | 1060 | 1020 | 3010 | 5270 | 5030 | 4850 | 15150 |
| 900 | 800 | 740 | 780 | 2320 | 3960 | 3630 | 3650 | 11240 |
| 1000 | 610 | 580 | 710 | 1940 | 3050 | 3315 | 3160 | 9525 |
| Total | 46419 | Total | 181840 | |||||
3. Summary for Light Fuel
Atomization improved according to relating numbers of droplets in evenly timed tests in the average for a fuel of 200 sec/Redwood 1 at 50°C.
The equal amount of fuel injected will show a 3.9 times finer droplet pattern 3.9 times more droplets containing the same amount of fuel.
HEAVY FUEL
| TIMING SEC. | LIGHT FUEL UNTREATED NO. OF DROPLETS |
LIGHT FUEL TREATED NO. OF DROPLETS |
||||||
| Test 1 | Test 2 | Test 3 | Total | Test 1 | Test 2 | Test 3 | Total | |
| 1/100 | 760 | 780 | 730 | 2270 | 4860 | 4620 | 4715 | 14195 |
| 200 | 650 | 640 | 610 | 1900 | 4210 | 3830 | 4220 | 12260 |
| 300 | 590 | 590 | 515 | 1695 | 3600 | 3415 | 3350 | 10365 |
| 400 | 430 | 400 | 410 | 1240 | 3490 | 3510 | 3560 | 10560 |
| 500 | 380 | 350 | 370 | 1100 | 2970 | 2760 | 2860 | 8590 |
| 600 | 290 | 280 | 260 | 830 | 2100 | 1940 | 1740 | 5780 |
| 700 | 180 | 210 | 210 | 600 | 1770 | 1840 | 1630 | 5240 |
| 800 | 90 | 110 | 80 | 280 | 1260 | 1350 | 1270 | 3880 |
| 900 | 60 | 120 | 110 | 290 | 830 | 910 | 860 | 2600 |
| 1000 | 70 | 110 | 90 | 270 | 515 | 470 | 460 | 1445 |
| Total | 10475 | Total | 74915 | |||||
4. Summary for Heavy Fuel
Atomization improved according to relating numbers of droplets in evenly timed tests, in the average for a fuel 1500 sec/Redwood 1 at 80°C
The equal amount of fuel injected will show after treatment under equal conditions as for untreated, an increase in the number of droplets average 7.2 times finer atomization.
5. Summary
By the use of Ivesco’s (Cataphore) for treatment of different types of fuel the atomization of fuel in the combustion chamber is substantially improved. Formation of smaller droplets makes these more equal sized. Readings were obtained by counting droplets per cm2 of a total screen area of 25 cm2.
Technical Specifications

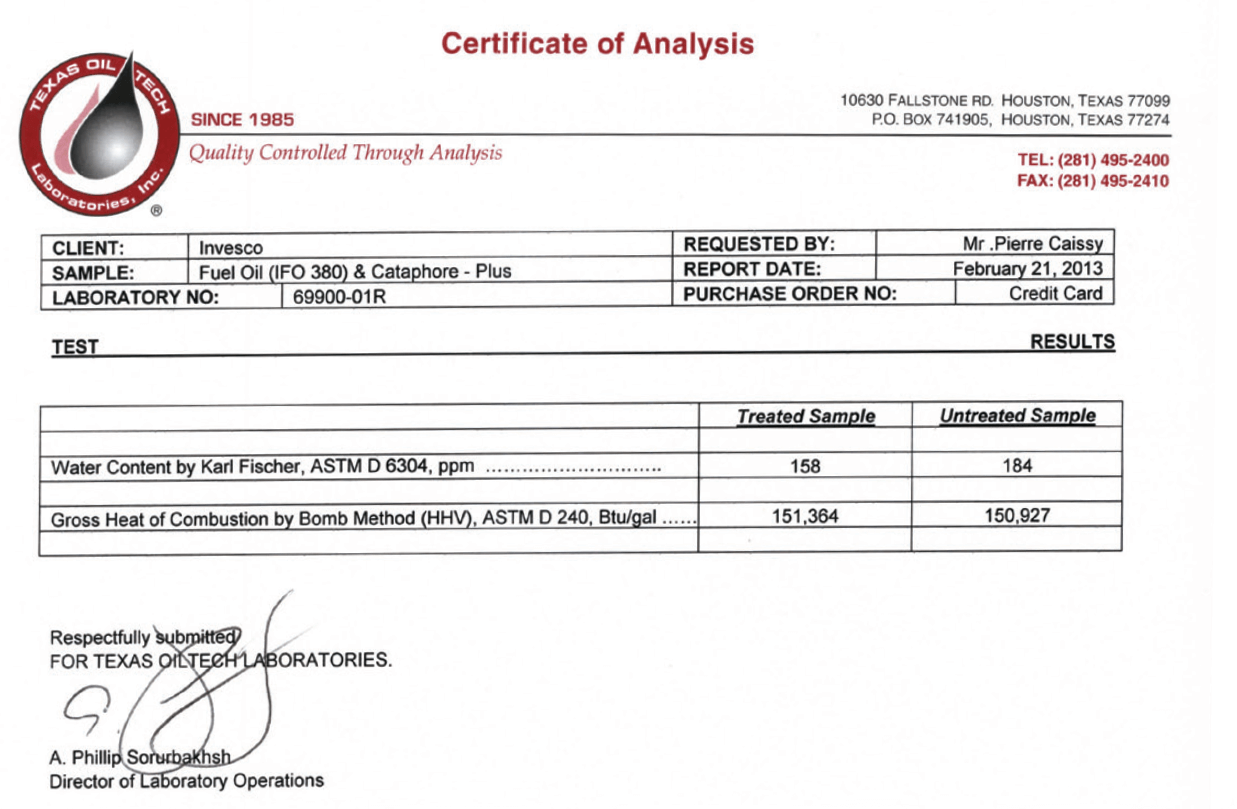
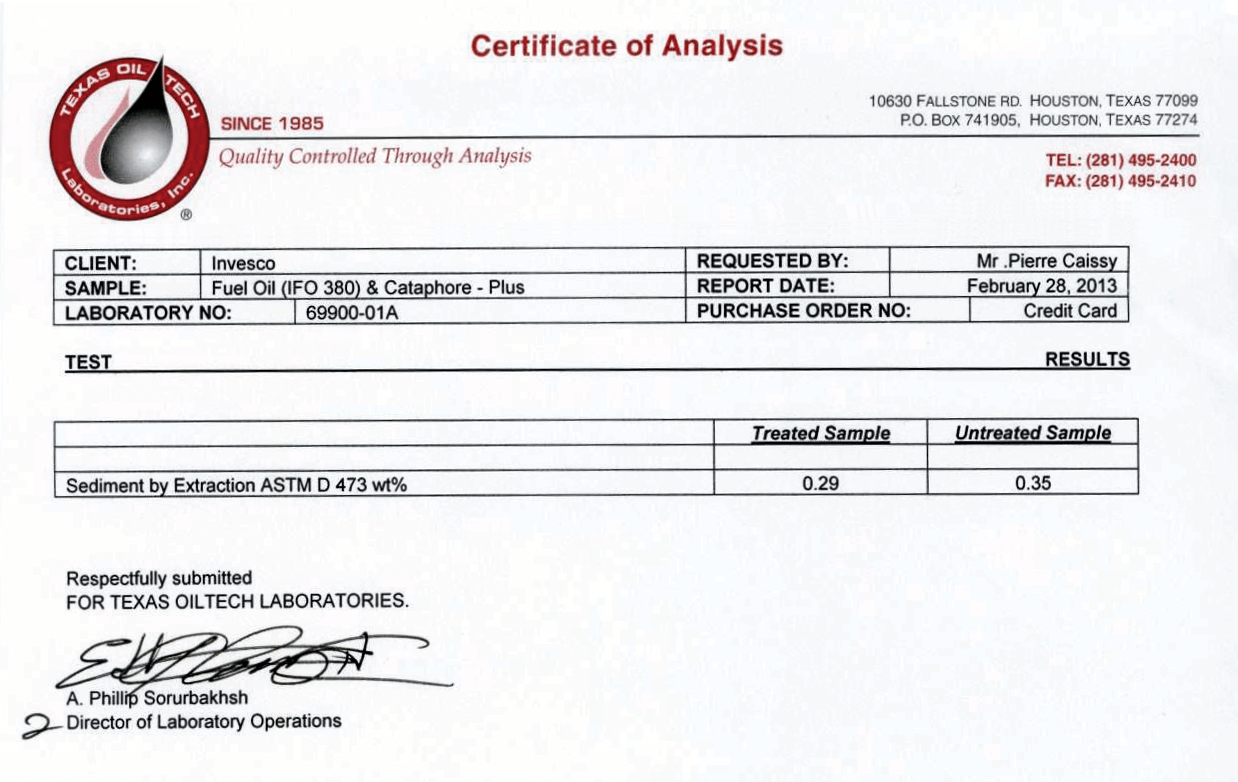
Certificates of Analysis
Letters of No Objection



